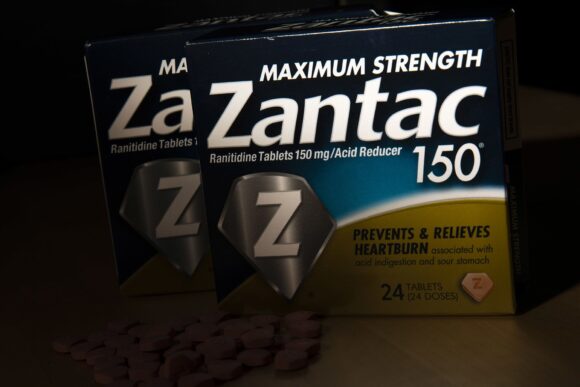
A jury in Chicago on Thursday rejected an Illinois woman’s claim that the now discontinued heartburn drug Zantac caused her colon cancer, in the first trial out of thousands of lawsuits making similar allegations.
The jury in Cook County, Illinois circuit court agreed with arguments from drugmakers GSK and Boehringer Ingelheim that the plaintiff, 89-year-old Illinois resident Angela Valadez, had not proven her colon cancer was at least in part caused by her Zantac use.
Valadez had alleged that her cancer was a result of taking over-the-counter Zantac and generic versions of it from 1995 to 2014. The lawsuits over the drug say its active ingredient, ranitidine, under some conditions turns into a cancer-causing substance called NDMA.
Attorneys for Valadez had asked the jury to award $640 million for her suffering. The judge rejected Valadez’s request to seek punitive damages during the trial, according to her attorneys.
Mikal Watts, one of Valadez’s attorneys, said he respected the jury’s verdict but was confident the companies would be held liable in future Zantac trials. “This is a marathon, not a sprint,” he said.
Both GSK and Boehringer said in statements that the verdict was consistent with scientific evidence that Zantac does not cause cancer, and that they would vigorously defend themselves against future claims.
Britain-based GSK, whose predecessor developed the drug but later sold the brand to other companies, and German drugmaker Boehringer Ingelheim, which sold the medicine from 2006 until 2017, were the only defendants in the trial after the other companies settled.
Watts said at the trial that began on May 2 that the companies knew that ranitidine would turn into NDMA as it aged or was exposed to extreme temperatures, but did not ensure it was properly handled by transporters, distributors and stores.
Attorneys for GSK and Boehringer countered that Zantac has been repeatedly proven to be safe and effective and that no scientific or medical study has connected Zantac to cancer.
The companies attorneys also argued at trial that there was no evidence to support Valadez’s claim that she had taken Zantac for 18 years, and that she had a host of risk factors that made her more likely to develop colon cancer.
The jury found that Valadez proved that she had taken Zantac, but not that it was a cause of her cancer.
First approved in 1983, Zantac became the world’s best selling medicine in 1988 and one of the first-ever to top $1 billion in annual sales.
In 2020, the U.S. Food and Drug Administration asked drugmakers to pull Zantac and its generic versions off the market after NDMA was found in samples of the drug. Thousands of lawsuits began piling up in federal and state courts.
The defendants notched a significant win in 2022, when a judge dismissed about 50,000 claims centralized in federal court in Florida. That judge concluded that the opinions of the plaintiffs’ expert witnesses that Zantac can cause cancer were not supported by sound science.
Some of the claimants in those cases are appealing the ruling to the Atlanta, Georgia-based 11th U.S. Circuit Court of Appeals.
A judge in Delaware state court is weighing the fate of about 72,000 cases, the bulk of those remaining, where the drugmakers similarly argue that plaintiffs’ expert testimony should be kept out.
Some other cases were previously settled, including several individual cases just before trial, and about 4,000 state court lawsuits outside of Delaware against Sanofi, which has owned the right to sell Zantac over the counter since 2017.
Earlier this month, the Financial Times reported that Pfizer had struck a deal to pay up to $250 million to settle more than 10,000 Zantac lawsuits.
Sanofi now sells Zantac360, a reformulated heartburn medicine whose active ingredient is famotidine.
Photo: Photographer: Drew Angerer/Getty Images
Topics Illinois
Was this article valuable?
Here are more articles you may enjoy.


 Insurance Broker Stocks Sink as AI App Sparks Disruption Fears
Insurance Broker Stocks Sink as AI App Sparks Disruption Fears  Florida Regulators Crack the Whip on Auto Warranty Firm, Fake Certificates of Insurance
Florida Regulators Crack the Whip on Auto Warranty Firm, Fake Certificates of Insurance  CFC Owners Said to Tap Banks for Sale, IPO of £5 Billion Insurer
CFC Owners Said to Tap Banks for Sale, IPO of £5 Billion Insurer  Munich Re Unit to Cut 1,000 Positions as AI Takes Over Jobs
Munich Re Unit to Cut 1,000 Positions as AI Takes Over Jobs 

